Bochet & Brewing Sugars - Reduction and Browning
Intentional browning of sugars has a long tradition in brewing and mead-making. Here's the science.

The Molecular Architecture of Sugar Reduction
In professional brewing and mead-making, the intentional thermal browning of sugars is a cornerstone of advanced flavor development. Whether crafting Belgian Candi Syrups or Bocheting Honey, you are manipulating the same fundamental pathways: breaking down complex sugars into simple ones (Inversion) followed by heat-induced browning (The Maillard Reaction).
This protocol details the molecular shifts occurring in sugar systems, the critical management of $pH$-drift, and the application of these substrates in both mead and beer, scaled to a 3.8-Liter (1-Gallon) equivalent.
Sugar Reductions take time, so if you want to experiment, make sure you have a good couple of hours to stand at a stove or burner!
A Note about Bochet
This is an ancient technique for creating a very dark mead with rich dark notes. Because modern methods avoid heating honey to retain delicate aromas and flavors, the idea of boiling or cooking honey might be controversial. Bochet is a very specific technique that intentionally changes honey character. That said, I'd avoid rare or exotic mono-floral honeys for this process, but darker and richer honeys intensify flavors when bocheted.
There are many modern bochet methods, storing in a heated space for slow darkening and maillard development, pressure cooking, the "crockpot" method, this article focuses on heating to the candy hard-crack phase and holding, a refinement of a cauldron of boiling honey over a fire.
Typically, Bochet falls into the "Experimental" mead category, but if using only honey, can also potentially be placed in Traditional if blended back to the same raw honeys to add just a touch of flavor and color. Blending back raw honey to the bocheted honey before fermentation helps to return appropriate aromatics and pure honey character to the mead. However, the flavors should support the declared honey varietal, even wildflower.
Bocheted honey pairs extremely well with dark stone fruits and warm spices and can often be that little something that pushes a great recipe into a stellar one.
A Note about Belgian Candi Sugar
Belgian Candi Sugar and British Brewing Sugars have a long, but more modern path. Both are also used both in brewing, candy making, and baking. There has been quite a bit of misinformation, likely due to protecting proprietary recipes and processes, and those Belgian Candi chunks from most brew supplies are really not the same, or at the least, don't have the added water to create a syrup, and are not truly inverted.
Tradition remains that monks used beet sugar (unrefined) and not sugar cane. Chemically, the only differences are the minor constituents from the sources that present nuanced flavor profiles. These sugars are primarily sucrose, result in a balance of glucose and fructose, and less sweat than honey.
The color variations are the result of time spent browning under heat and acid conditions. I've had good results using a blend of white sugar and light brown sugar, but also have used demonara and even piloncillo sugars to emphasize different flavors.
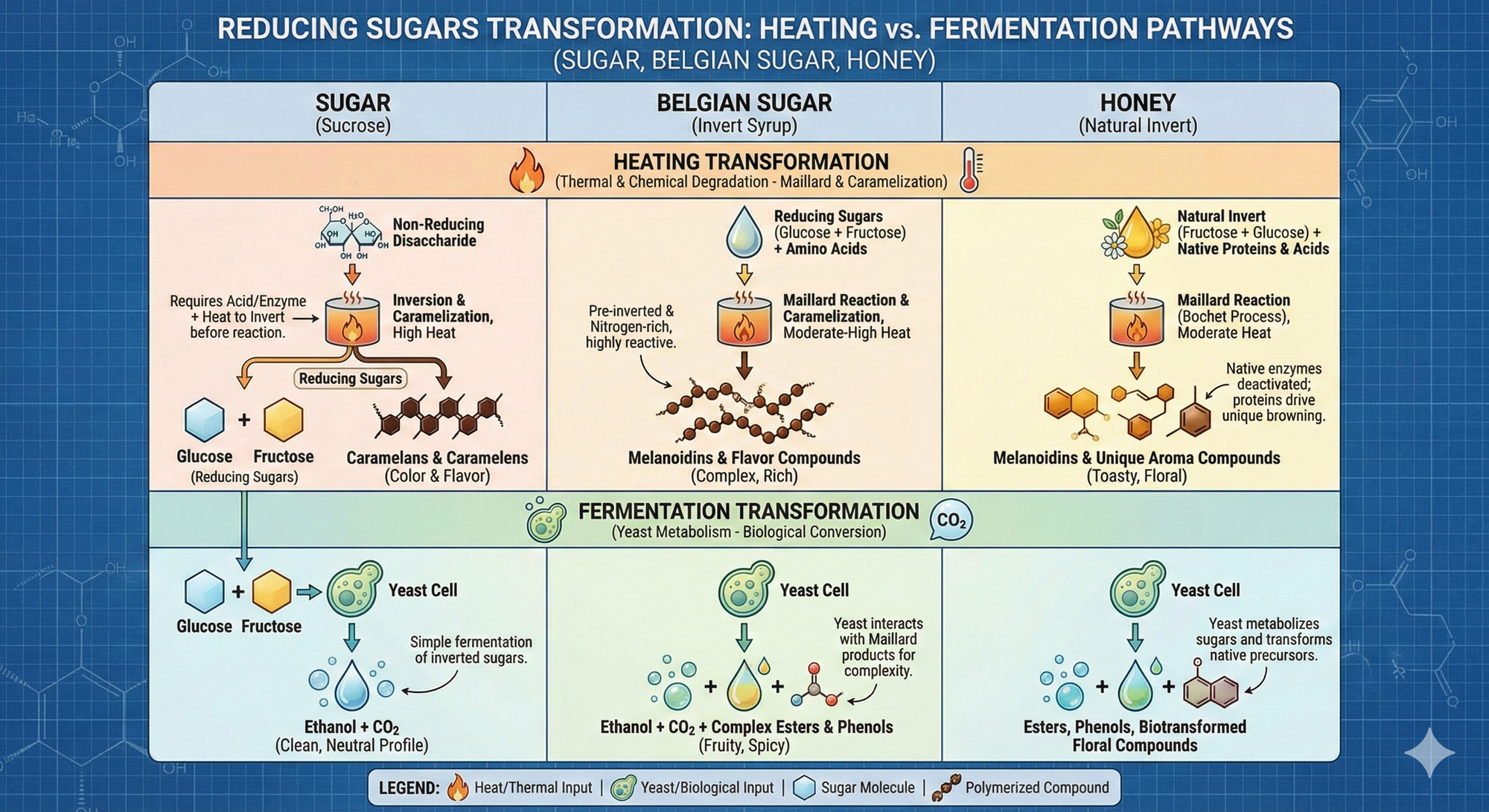
The Chemical Foundation: Inversion & Browning Pathways
Before browning begins, the sugar must be in its "reducing" state, requiring the cleavage of complex sugars into simple ones.
The Gatekeeper: Inversion
-
Sucrose (Table Sugar/Beet Sugar): This is a "non-reducing" disaccharide. It is stable under heat and will not brown effectively until it is "inverted" (hydrolyzed) into its constituent monosaccharides: Glucose and Fructose.
-
The Process: This is achieved using heat and an acid catalyst:
- Citric Acid: $C_{6}H_{8}O_{7}$
- Cream of Tartar (Potassium Bitartrate): $KC_{4}H_{5}O_{6}$
-
The Reaction:
$$C_{12}H_{22}O_{11} + H_{2}O \xrightarrow{\text{Acid/Heat}} C_{6}H_{12}O_{6} + C_{6}H_{12}O_{6}$$ -
Raw Honey: Honey is uniquely "pre-inverted" by the honeybee via the enzyme Invertase, but a little sucrose might remain. Honey enters the process already in a reactive state, allowing browning to initiate almost immediately at 100°C (212°F).
The Heyns Pathway
While beer wort follows a specific browning route, fructose-heavy systems like honey and invert syrups utilize the Heyns Pathway. In the presence of nitrogen (found naturally in honey or added via nutrients like Diammonium Phosphate, Fructose reacts to form specific flavor compounds. Tfis is the primary engine for the "toasted marshmallow," "dark cocoa," and "biscuit" profile.
A Note about Inverts
Because the carbon bonds of sucrose have been cleaved, inverts (glucose and fructose) can be immediately consumed by yeast, and may provide less stress during fermentation, provided adequate or optimal available nutrients. This means that one should consider the fermentation approach and adjust for a faster and more vigorous ferment.
With pure sucrose, the yeast must produce enzymes to break those carbonic bonds. This may stall vigorous fermentation and increase lag times. I cannot find any credible sources on the impact on off-flavor production from yeast stress, but it makes sense that if yeast use nutrients in the process of enzyme production, that energy is not available for anaerobic alcohol production, or there maybe more yeast die-off as nutrients are depleted.
Some rational experimentation is needed to confirm my hypothesis, but always, do what works for you to produce the best beer/mead/cider that you enjoy. I'm just some noise on the internet.
Technical SOP: The Sugar Reduction Process
This process requires some consideration before attempting. Boiling sugar syrups will expand dramatically during the boiling process, and "spit" lava-hot droplets. Use a large pot with high walls, and always wear gloves. The steam generation from quenching can also burn, so stand away and be very careful. I have a 5 gallon pot, but never attempt more than 2 gallons of syrup at a time.
Heating gently and slowly to temps will minimize scortching and allow you to target temps very specifically. A high quality candy thermometer or a high-temp digital thermometer is necessary. Given this is a 500 ml recipe - scale appropropriately to your volume/mass requirement.
Step 1: Loading the Reactor
- Substrate: Use 400g of honey or sugar.
- Initial Water: Add 100ml of water to the pot first.
- Incorporate: Stir and dissolve fully before applying heat.
Step 2: pH Management & The "Room Temp" Rule
As sugar browns, it creates organic acids—primarily Acetic Acid ($CH_{3}COOH$) and Formic Acid ($HCOOH$)—that cause the $pH$ to drop.
- The Correction: Extract a 10-20 ml sample, cool to 25°C (77°F) in an ice bath.
- The Fix: If the sample is below pH 5.5, add 1g of Potassium Carbonate ($K_{2}CO_{3}$) to the main pot to buffer the acidity.
Step 3: The Thermal Hold (140°C)
- Target: Reach 140°C (284°F). Go slowly and heat gently to target to allow water to escape at lower temperatures to avoid lava-like spitting.
- Maintenance: Hold for 60 minutes. Stir constantly to prevent carbonization (burning).
Step 4: The Quench
- Ratio: Use 200ml (2:1 ratio to initial water) of quench water.
- Temperature: Heat quench water to 90°C to avoid thermal shock/splattering.
- Action: Pour slowly into the 140°C syrup. Warning: Massive Steam Output.
Experimental Recipes (3.8L / 1-Gallon Scale)
A. The "Tri-Sugar" Experimental Mead
- The Blend: 400g Bochet Honey + 800g Raw Honey + 100g Dark Belgian Candi Syrup.
- Yeast: Lalvin 71B (3g–5g).
- Nutrient: 5.6g Fermaid O (Split into 4 additions of 1.4g).
- Expectation: OG: 1.125 | FG: 1.015.
B. The "Heyns-Invert" Imperial Porter (Beer)
- Grist: 1.2kg Maris Otter, 100g Brown Malt, 50g Black Malt.
- Additions: 200g Raw Honey (Flameout), 100g Dark Candi Syrup (15m).
- Protection: 2g Opti-White at pitch (Glutathione source).
Aging, Fining, and Stability
Clarity
Cooked honey often creates a stubborn colloidal haze and releases a good amount of dusting during aging.
- Fining: Use Kieselsol and Chitosan or other fining agents post-fermentation.
- Filtration: 1.0 or 0.5 micron filter for a professional polish.
Maturation
- Bulk Aging: 6–12 months in a carboy to soften "harsh" edges into leather and dark fruit. This also allows any remaining powdery byproducts to fall out of solution, and gluconic acid to emerge.
- Oak: Add 5g–10g of Oak Cubes for structural tannins.
Stabilization
Use both metabisulfite and potassium sorbates to stabilize and final rack 24-48 hours before packaging.
Technical Summary Matrix
| Compound Group | Sensory Profile | Stage of Development |
|---|---|---|
| Maltols | Toasted Marshmallow / Cotton Candy | 120°C – 140°C |
| Pyrazines | Dark Chocolate / Roasted Nuts | High-Heat Maillard (140°C+) |
| Furans | Burnt Pineapple / Strawberry | Fructose Breakdown |
| Melanoidins | Bread Crust / Cocoa / Body | High-Order Polymerization |
Authoritative References
- Kunze, W. (2014). Technology Brewing and Malting.
- Belitz, H. D. (2009). Food Chemistry.
- White, J. W. (1975). Composition of Honey.
- Hodge, J. E. (1953). Chemistry of Browning Reactions.